Designing Efficient Catalysts
The growing demand for energy and the concurrent increase in pollution has underscored the urgent need for renewable and clean energy sources. To this end, various chemical reactions, including water splitting, carbon dioxide reduction, and nitrogen reduction, have been investigated for the production of renewable fuels such as hydrogen and methane, as well as for pollutant decomposition and chemical processing. However, many of these reactions are non-spontaneous and necessitate the use of catalysts for their feasibility. The focus of our group is towards developing design principles for a new class of state-of-the-art catalysts using both first-principles and machine learning approaches. One of the most widely accepted techniques to decrease high levels of CO in the atmosphere is the CO oxidation to CO2. We have observed high activity for CO oxidation by varying the size of a single-layered Ptn cluster supported on the non-reducible Si (111)-7×7 [1]. This alteration positions the d-band center of the Pt cluster suitably for optimum adsorption of reactants, CO and O2.
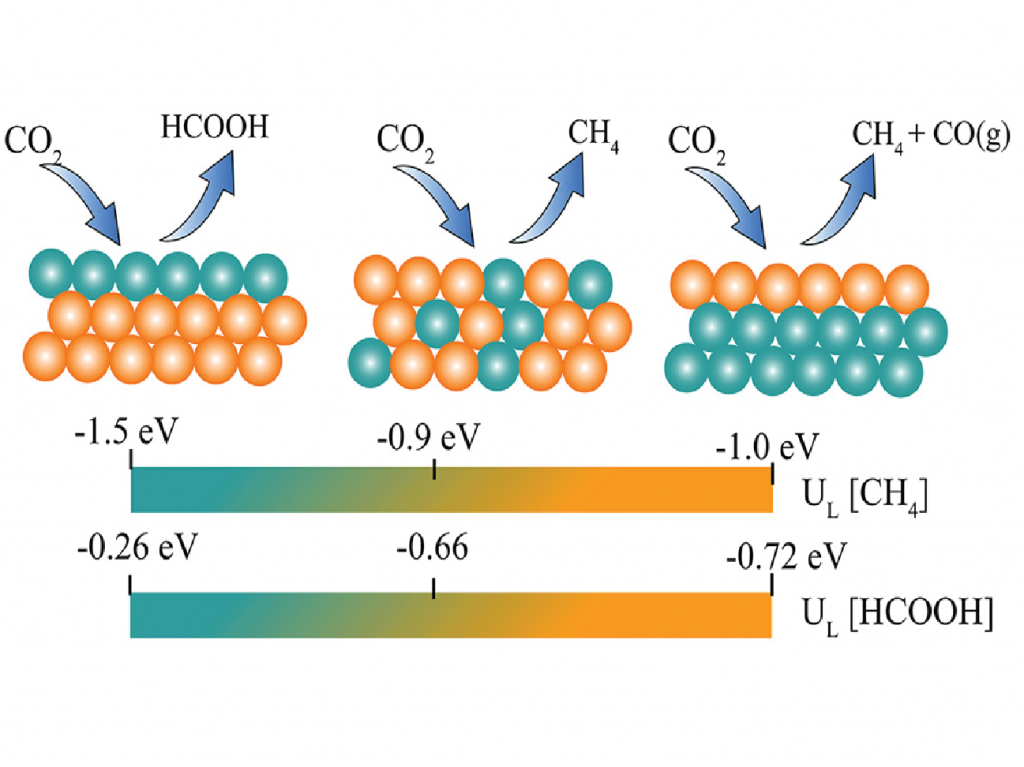
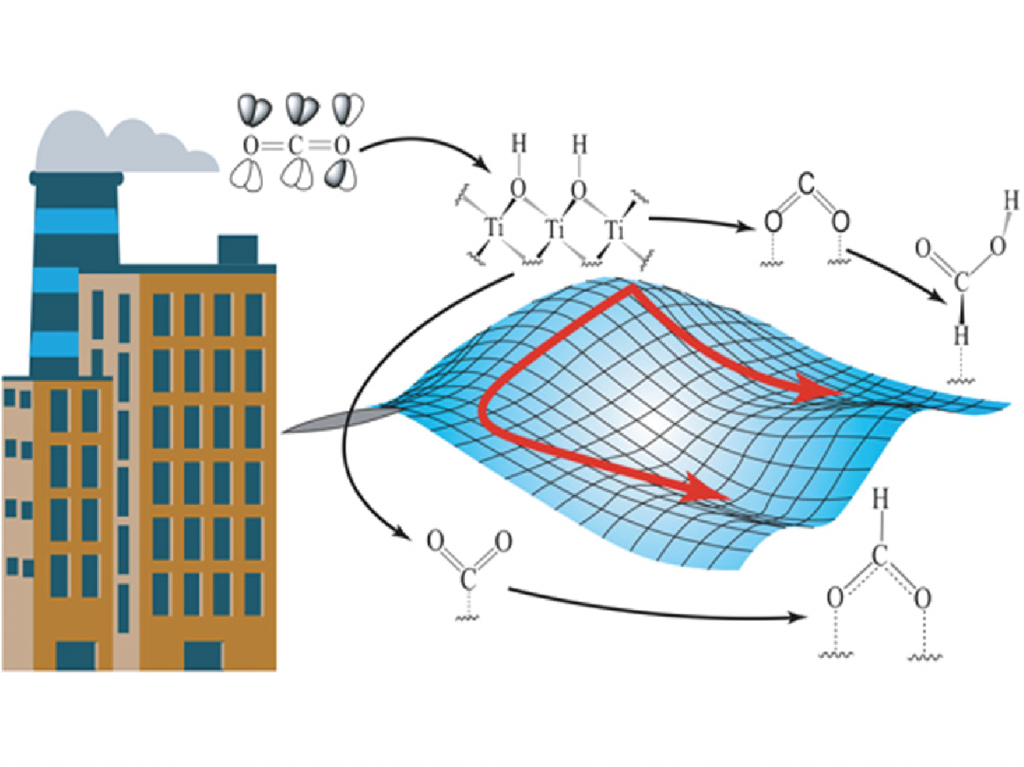
The electroreduction of CO2 gas to valuable chemicals like formic acid and methane exhibits great promise in mitigating CO2 from the atmosphere. To build a suitable catalyst for this purpose, we tuned the composition of the surface elements with Pd and Au to generate different important intermediates, which confirmed an increasing selectivity towards methane rather than formic acid with increasing Au-concentration on the surface [2]. Au3Pd shows maximum activity for yielding methane with a very low limiting potential of -0.9V vs SHE. The excess energy required to convert CO2 to useful products can possibly be eliminated by using Ti2C(OH)2 MXene [3]. This is because adsorbed CO2 over Ti2C(OH)2 surface spontaneously crosses the activation barrier involved in the formation of multiple intermediates to finally yield formic acid. Such spontaneity is observed as metallic Ti2C(OH)2 contains easily donatable protons and high energy electrons near the Fermi level.
To achieve spontaneity for N2 reduction to NH3 in direct sustainable fuel production, which is a great alternative to pollution abatement, we anchored 13 transition metals on a well-known MoS2 monolayer and compared them for N2 reduction to NH3 [4]. The highest activity can be achieved by considering two factors: charge transferred from the transition metal to N2 molecule and group number of transition metals. Based on this understanding, a search scheme was further employed, suggesting Mo- and W-C3N4 as the most suitable N2 reduction catalysts [5].
Photocatalytic reactions can be considered another great alternative source of pollution-free clean energy. To make a suitable photocatalyst, the major criteria are the visible range band-gap and suitable band-edge positions. This can be achieved perfectly in C2N/WS2 van der Waals heterostructures in which due to charge transfer between layers enables the separation of the electrons and holes in two different layers and increases their lifetime [6].
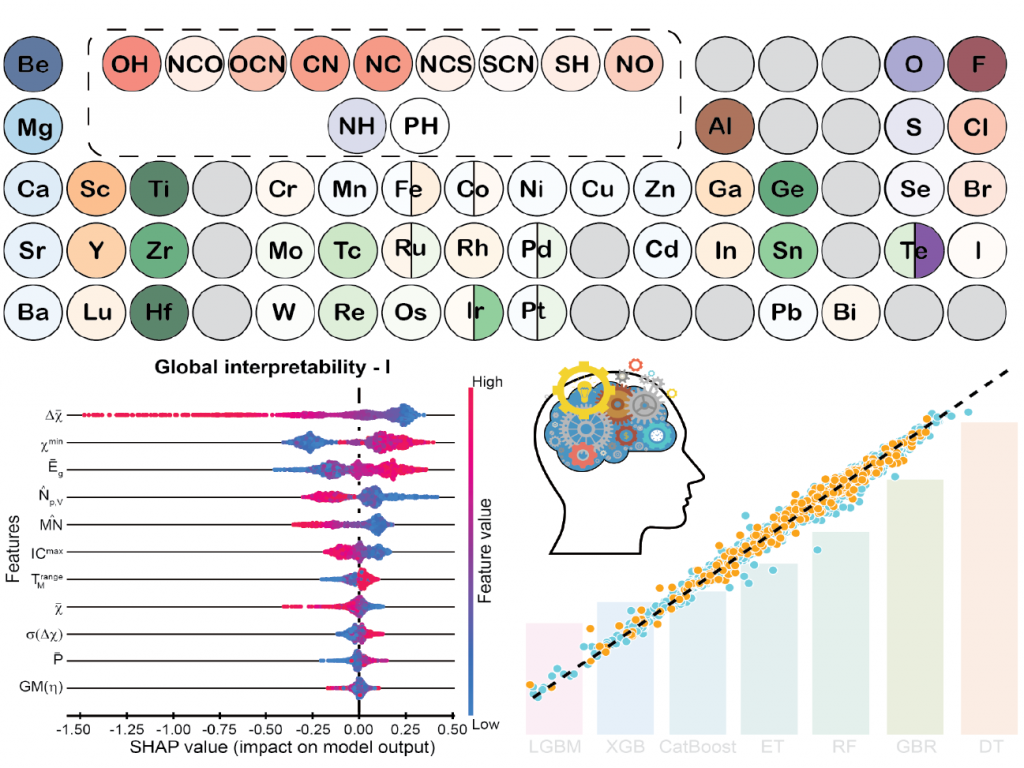
However, searching through all the possible 2D photocatalysts and understanding their stability and property is time-consuming. Therefore, we devised an intuitive method of interpreting such opaque ML models through SHapley Additive exPlanations (SHAP) values and coupling them with the HT approach for finding efficient 2D water-splitting photocatalysts [7]. The interpretable ML regression, classification, and data analysis lead to a new hypothesis that highly stable 2DO materials follow the HSAB principle. Moreover, HfSe2 and ZrSe2 were found to have high solar-to-hydrogen efficiencies reaching their theoretical limits.
Refrences
- 1. R. Ahmad, H. Yasumatsu, and A. K. Singh, Highly Efficient CO Oxidation on Atomically Thin Pt Plates Supported on Irreducible Si 7×7, J. Phys. Chem. C, 127, 4527 (2023)
- 2. S. Agarwal, and A. K. Singh, Electroreduction of CO₂ with Tunable Selectivity on Au-Pd Bimetallic Catalyst: A First Principle Study, ACS Appl. Mater. Interfaces, 14, 11313-11321 (2022)
- 3. A. Parui, P. Srivastava, and A. K. Singh, Selective Reduction of CO₂ on Ti₂C(OH)₂ MXene through Spontaneous Crossing of Transition States, ACS Appl. Mater. Interfaces, 14, 40913-40920 (2022)
- 4. R. Kumar, and A. K. Singh, Electronic Structure Based Intuitive Design Principle of Single‐Atom Catalysts for Efficient Electrolytic Nitrogen Reduction, ChemCatChem, 12, 5456-5464 (2020)
- 5. S. Agarwal, R. Kumar, R. Arya, and A. K. Singh, Rational Design of Single-Atom Catalysts for Enhanced Electrocatalytic Nitrogen Reduction Reaction, J. Phys. Chem. C, 125, 12585-12593 (2021)
- 6. R. Kumar, D. Das, and A. K. Singh, C2N/WS2 Van der Waals Type-II Heterostructure as a Promising Water Splitting Photocatalyst J. Catal. 359, 143 (2018)
- 7. R. Kumar, and A. K. Singh, Chemical Hardness-driven Interpretable Machine Learning Approach for Rapid Search of Photocatalysts, npj Comput. Mater., 7, 197 (2021)
- 8. R. Ahmad and A. K. Singh, Simultaneous Site Adsorption Shift and Efficient CO Oxidation Induced by V and Co in Pt Catalyst J. Phys. Chem. C 121, 12807 (2017)
- 9. R. Ahmad, and A. K. Singh, Pt Poisoning Free Efficient CO Oxidation on Pt3Co Supported on MgO(100): An Ab Initio Study, ACS Catalysis 5, 1826 (2015)